Get the Big Picture view of campus safety
CampusOptics is a cross-functional EH&S platform designed specifically for institutions of higher education. CampusOptics was created to help campus safety professionals improve collaboration, reduce institutional risk and enhance safety culture.
An EH&S solution that is as Mobile as you are
EH&S Professionals are almost never at their desks, which is why CampusOptics offers a mobile app for both IOS and Android devices to support on-the-go access to chemical inventory, hazardous waste containers, inspections, safety assets, incident records and emergency plans.
Barcode & QR code scanning
Use your device’s camera to scan bar/QR codes for key information on safety assets or chemical containers.
In-App Photos and Video
Quickly associate pictures and videos to assets, chemical containers, safety issues and inspection reports.

Talk-to-Text
Streamlin
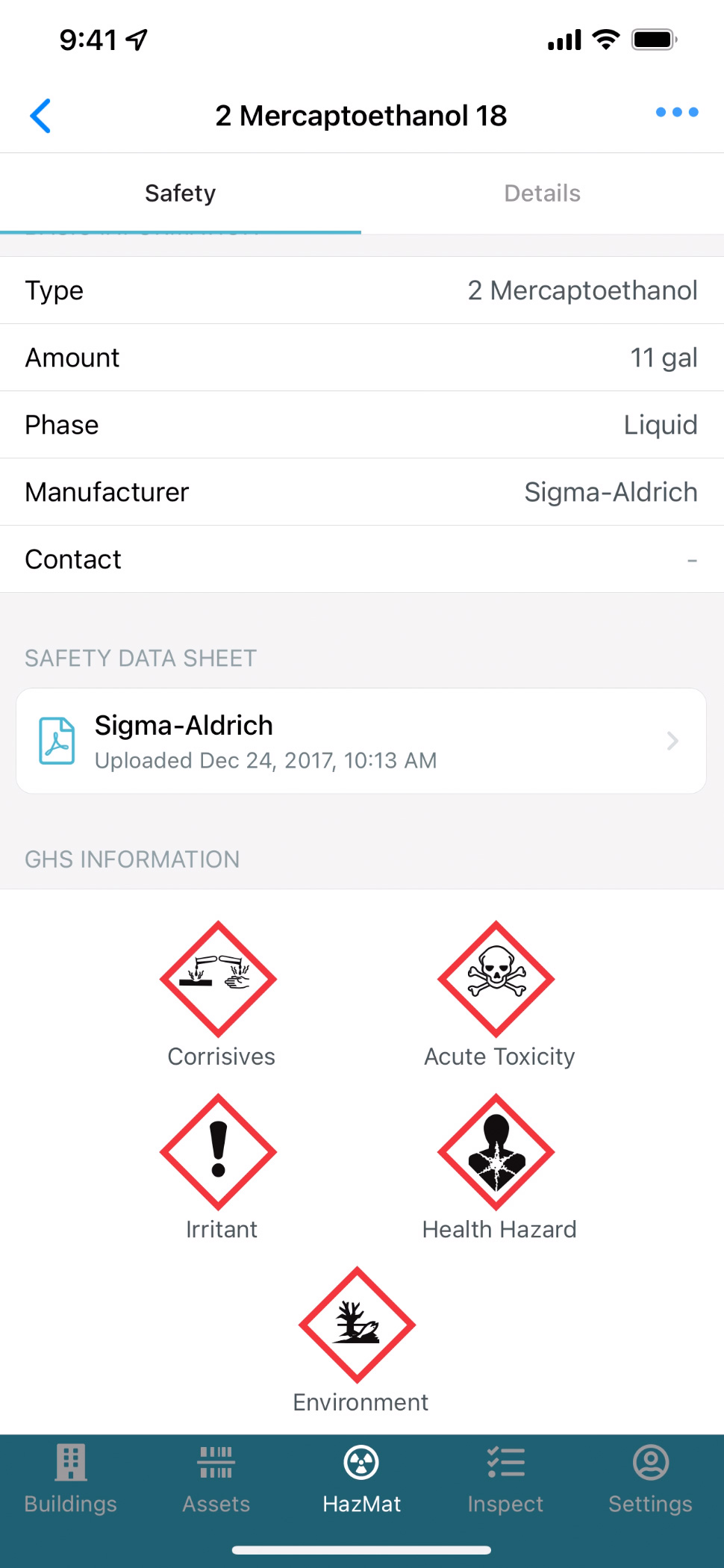
Access Documents
Access key documents on your mobile device, including Safety Data Sheets, emergency plans, floor plans and product documentation.

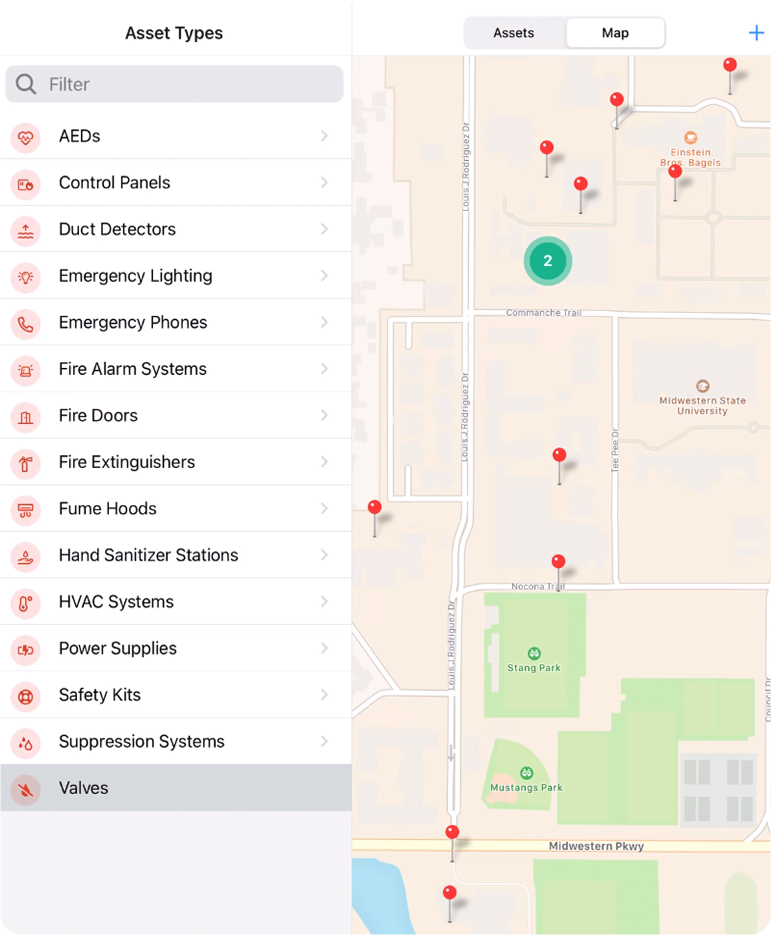
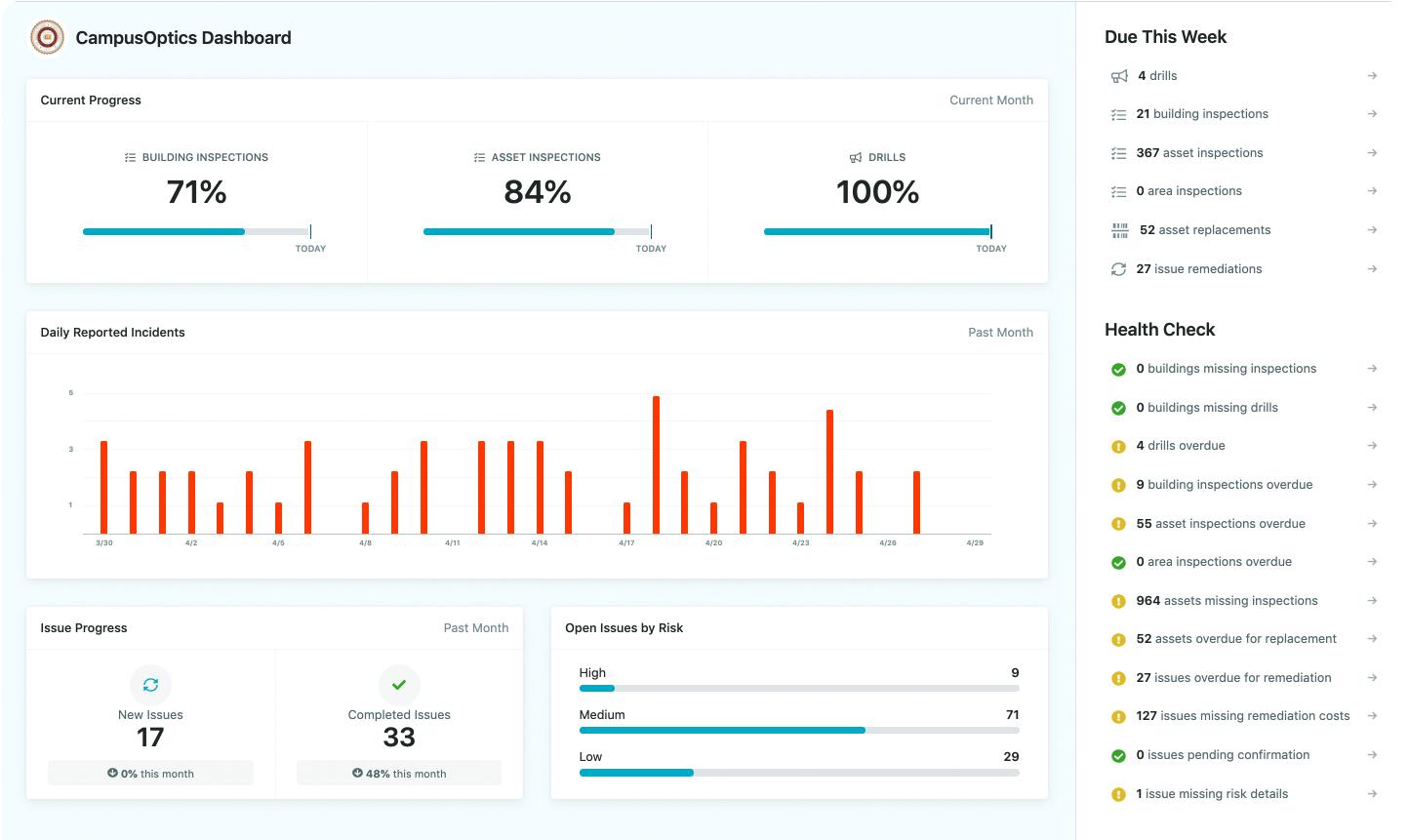
Visualize your data
View the location of safety assets, chemical containers, incidents and safety related issues across campus. Generate public facing maps of key assets like AED’s and Blue Phones.
Locations can be mapped automatically via import or you can log coordinates using your mobile device.
Get the Big Picture view of campus safety
CampusOptics is a cross-functional EH&S platform designed specifically for institutions of higher education. CampusOptics was created to help campus safety professionals improve collaboration, reduce institutional risk and enhance safety culture.
Pharmaceutical compliance is no longer just about passing inspections – it’s about ensuring patient safety, protecting supply chains, and meeting complex global regulations. From FDA and EMA requirements to GMP, GLP, and GxP standards, life sciences organizations operate under constant scrutiny that demands structured, reliable oversight.
Pharmaceutical compliance software provides the tools to manage these processes in one place, replacing fragmented paper records and manual spreadsheets with digital platforms built for accuracy and traceability. With features like audit trails, electronic signatures, automated workflows, and real-time reporting, teams can document compliance activities, track quality events, and stay aligned with evolving regulatory expectations. This approach shifts compliance from a reactive burden into a proactive framework that strengthens trust, reduces risk, and supports innovation in the pharmaceutical industry.
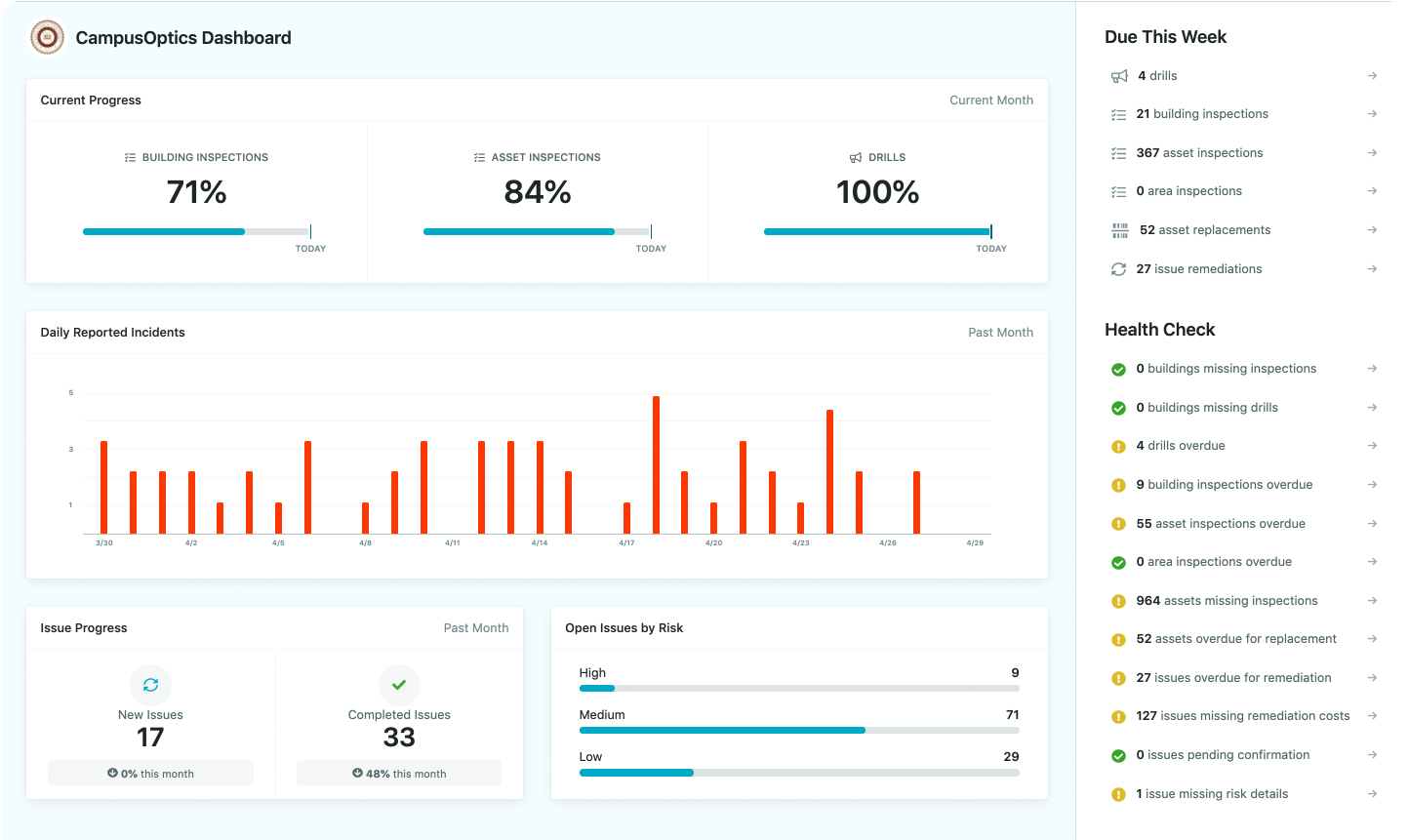
1. CampusOptics
CampusOptics provides a compliance platform built for higher education institutions that need structured oversight of environmental health, safety, and fire and life safety activities. We centralize chemical inventory, hazardous waste management, inspections, and radiation oversight, while also supporting functions such as incident reporting, training assignments, and compliance calendars. Although developed for universities, it can also be applied as pharmaceutical compliance software, helping laboratories and life sciences organizations manage chemical safety, hazardous waste streams, and regulatory reporting in line with industry standards.
Key Highlights:
- Built specifically for higher education compliance and safety needs
- Also adaptable as pharmaceutical compliance software for labs and life sciences
- Centralized platform for chemical inventory, waste, inspections, and permits
- Mobile app with barcode/QR code scanning, media capture, and talk-to-text input
- Tools for emergency planning, incident management, and training assignments
- Configurable permissions with single sign-on integration
Services:
- Chemical inventory and safety data sheet (SDS) management
- Hazardous waste tracking and manifest oversight
- Laboratory and facility inspections with remediation workflows
- Permit creation and approval for high-risk activities
- Incident reporting and investigation logging
- Occupational safety management and training assignments
- Compliance calendar for tasks and regulatory deadlines
- Support for EHS, risk management, fire safety, and emergency planning teams
- Use in pharmaceutical environments for GxP-aligned tracking and compliance reporting
Contact Information:
- Website: www.campusoptics.com
- App Store: apps.apple.com/ua/app/campusoptics
- Google Play: play.google.com/store/apps/campusoptics
- Address: 7951 Shoal Creek Blvd., Suite 275 Austin, TX 78757
- Phone Number: (888)748-7652
- Email: contact@campusoptics.com
2. SAI360
SAI360 presents a governance, risk, and compliance platform that connects policy management, compliance training, and risk processes in a single system. The platform is structured to unify governance across functions, link ethics and compliance activities, and centralize administration for policy, controls, and reporting. For pharmaceutical use, the approach supports consistent execution of compliance tasks, visibility across operations, and coordination with changing regulations without relying on separate tools.
The platform provides configurable modules and use cases that align to varied regulatory frameworks. Program owners can coordinate third party oversight, policy rollout, incident workflows, and audit readiness from one place. Dashboards and behavioral analytics supply context for monitoring and improvement, while industry solutions help adapt controls and documentation to pharmaceutical requirements.
Key Highlights:
- Integrated GRC platform for policy, risk, and compliance programs
- Configurable modules to match program scope and maturity
- Connected policy administration, training, and compliance management
- Dashboards and behavioral analytics for oversight
- Industry solution sets for regulated environments
Services:
- Compliance policy lifecycle and training management
- Risk and control management with monitoring and reporting
- Incident and case tracking with remediation workflows
- Third party and due diligence oversight
- Program dashboards, analytics, and documentation support
Contact Information:
- Website: www.sai360.com
- LinkedIn: www.linkedin.com/company/sai360
- Address: 205 West Wacker Drive Suite 1800 Chicago, IL 60606 United States
- Phone Number: +1 (312) 546-4500
3. GAN Integrity
GAN Integrity provides a connected compliance platform focused on unifying ethics, compliance, and third party risk in one environment. The system brings disclosures, gifts and entertainment, due diligence, conflicts of interest, training, policy management, and investigations under a single data model. For pharmaceutical programs, this structure supports anti bribery and corruption requirements, supply chain due diligence, and documentation for global regulations without moving between separate systems.
The platform includes products for third party risk management and regulatory alignment across frameworks such as FCPA, UK Bribery Act, EU Whistleblower Directive, and supply chain acts. Workflows and integrations help screen and onboard third parties, assess and monitor risks, and manage remediation. Policy and training tools coordinate expectations across teams, while case management and reporting provide traceable outcomes.
Key Highlights:
- Unified environment for ethics, compliance, and third party risk
- Products for disclosures, gifts, conflicts, and investigations
- Regulatory alignment for anti corruption and supply chain laws
- Workflows and integrations for screening, assessments, and monitoring
- Centralized policy and training management with reporting
Services:
- Third party due diligence and ongoing risk monitoring
- Policy management, attestations, and training delivery
- Disclosures for conflicts, gifts, and sponsorships
- Case and incident management with remediation tracking
- Reporting and documentation to support audits and reviews
Contact Information:
- Website: www.ganintegrity.com
- LinkedIn: www.linkedin.com/company/ganintegrity
- Address: 228 Park Ave S, PMB 44939 New York, NY 10003 United States of America
4. VComply
VComply offers a compliance and risk operating system that converts policies and controls into assigned, trackable tasks. The platform consolidates activities like policy approvals, control checks, incident follow up, and evidence collection in one place. For pharmaceutical compliance, this task centered approach supports routine execution, accountability, and quick access to audit ready records across sites and functions.
The system emphasizes workflow automation, calendar and alerts, dashboards, and mobile access. Modules cover policy lifecycle, risk assessment with heatmaps, incident and corrective action tracking, and site and safety audits. No code setup and role based access help teams standardize execution without heavy IT effort, while exports and reports provide structured outputs for inspections and audits.
Key Highlights:
- Task based compliance execution across policies, controls, and audits
- Workflow automation with reminders, escalations, and evidence capture
- Dashboards and reports to track status and readiness
- Policy lifecycle, risk assessments, and incident tracking
- Mobile first access for distributed teams and sites
Services:
- Policy creation, approvals, acknowledgments, and training assessments
- Control task scheduling, ownership, and audit trail management
- Risk evaluations with heatmaps and review workflows
- Incident and corrective action tracking with follow up
- Site and safety audits with standardized templates and reporting
Contact Information:
- Website: www.v-comply.com
- LinkedIn: www.linkedin.com/company/vcomply
- Address: 440 N Wolfe Rd, Sunnyvale, California-94085, USA
- Email: help@v-comply.com
5. SoftExpert
SoftExpert is an integrated governance, risk, and quality platform that brings documents, workflows, audits, and risks into one environment. The software includes broad compliance tooling across QMS, GRC, ECM, EHSM, ERM, PLM, BPM, supplier lifecycle, and more. For pharmaceutical use, the platform supports alignment with key regulations and standards such as FDA 21 CFR Part 11 and Part 820, ISO 13485, ISO 14971, and related frameworks. The mix of controlled documents, training, and change tracking helps keep procedures consistent and traceable across sites and teams.
The platform’s module set covers daily execution and oversight. Projects, tasks, and workflows handle routine work, while risks, audits, and KPIs add structure for monitoring and improvement. Content management supports secure storage and controlled access, with version history to preserve records. EHS and quality modules address incidents, corrective actions, and verification activities, so compliance tasks do not sit apart from operations.
Key Highlights:
- Unified suite spanning QMS, GRC, ECM, EHSM, ERM, PLM, BPM, and supplier lifecycle
- Support for FDA 21 CFR Part 11 and Part 820, ISO 13485, ISO 14971, and related standards
- Centralized document control with workflows, permissions, and version history
- Risk, audit, CAPA, and KPI features connected to everyday processes
- No code workflow automation for approvals, requests, and recurring tasks
Services:
- Quality management with document control, training, and change control
- Governance and risk management with assessments, controls, and reporting
- Audit planning and execution with findings, actions, and evidence tracking
- EHS management for incidents, permits, KPIs, and corrective actions
- Enterprise content management for secure, compliant recordkeeping
Contact Information:
- Website: www.softexpert.com
- LinkedIn: www.linkedin.com/company/softexpert-software
- Phone Number: +1 (321) 442-7145
- Facebook: www.facebook.com/SoftExpertSoftwareforPerformanceExcellence
- Twitter: x.com/softexpert
- Instagram: www.instagram.com/softexpert
- Email: salesturkey@softexpert.com
6. SimplerQMS
SimplerQMS is a life sciences quality management system that consolidates core quality processes in a single cloud platform. Modules include document control, training, change control, deviation and nonconformance handling, CAPA, complaints, audits, risk, equipment, supplier management, product and batch records. The software is designed for regulated environments in pharmaceuticals, biotech, medical devices, laboratories, CRO and CMO operations, with validation and audit readiness built into the approach.
Compliance alignment covers GxP and standards such as FDA 21 CFR Parts 210, 211, and 820, EU GMP Annex 11, ISO 13485, ISO 9001, ICH Q10, and related guidance. Microsoft 365 integration allows document editing while maintaining an audit trail. Prevalidated deployment and ongoing validation evidence reduce overhead for qualification activities. The result is a consistent set of connected workflows that keep documentation, training, and corrective actions tied to real work, not scattered across tools.
Key Highlights:
- End to end QMS modules for regulated life sciences environments
- Alignment with GxP, FDA 21 CFR Parts 210, 211, 820, EU GMP Annex 11, ISO 13485, and ICH Q10
- Built in validation according to GAMP5 with audit trail and controlled records
- Microsoft 365 integration for authoring with compliance safeguards
- Standardized workflows for changes, CAPA, deviations, and audits
Services:
- Document control with versioning, e-signatures, and controlled approvals
- Training management with assignments, reminders, and acknowledgment tracking
- Change control, deviation, nonconformance, and CAPA workflows
- Complaint handling with linked investigations and outcomes
- Audit and risk management with plans, findings, and mitigation tracking
- Equipment and supplier management with calibration and qualification records
- Electronic batch and product records for traceable manufacturing documentation
Contact Information:
- Website: simplerqms.com
- LinkedIn: www.linkedin.com/company/simplerqms
- Address: 600 B St, San Diego, CA 92101, United States
- Phone Number: +18888309807
7. AskGxP
AskGxP is an AI driven knowledge and compliance assistant focused on pharmaceutical GxP needs. The platform provides AI chat for regulatory guidance, AI generated SOPs and protocol drafts, and curated updates on relevant guidelines. Knowledge is organized to support quick lookups and practical answers, reducing time spent searching through reference documents and historical files.
For larger programs, the system extends into customized AI assistants trained on organization specific content. Learning tools support knowledge transfer and targeted upskilling, while analytics help surface frequent questions or gaps in understanding. The aim is to keep procedures, training, and day to day decisions aligned with current expectations, using AI to accelerate document creation and reduce ambiguity in routine compliance tasks.
Key Highlights:
- AI assistant for GxP questions, guidance, and quick lookups
- AI generated SOPs and protocol drafts to speed document authoring
- Curated regulatory updates to keep knowledge current
- Options for custom assistants trained on internal content
- Learning tools and insights to close knowledge gaps
Services:
- AI chat for GxP guidance and compliance interpretation
- SOP and protocol generation with structured templates
- Knowledge curation with ongoing updates and summaries
- Custom AI assistant configuration for enterprise use
- Training support with learning paths and skills development
Contact Information:
- Website: www.askgxp.com
- Address: 20 Harcourt Street,Dublin , Ireland
- Phone Number: +353 (01) 912 5373
- Email: info@askgxp.com
8. rfxcel
rfxcel is a supply chain traceability platform centered on pharmaceutical serialization, compliance tracking, and real-time visibility from manufacturing through distribution. The system connects product data, events, and partners to maintain end-to-end traceability, with tooling for regulatory reporting, EPCIS exchange, and alert handling. Environmental monitoring and status dashboards support ongoing oversight, helping operations confirm product integrity and chain-of-custody across sites and lanes.
For pharmaceutical programs, the platform aligns daily logistics with compliance controls, including serialization lifecycle management, exception handling, and audit-ready records. Integration options link to existing ERP, WMS, and packaging lines, while configurable workflows route investigations and remedial tasks. The practical outcome is fewer blind spots in movement, storage, and documentation, which reduces manual reconciliation and supports inspection readiness.
Key Highlights:
- End-to-end pharmaceutical serialization and traceability
- Regulatory reporting and EPCIS data exchange with partners
- Real-time status, alerts, and exception management
- Environmental monitoring for storage and transit conditions
- Integration with ERP, WMS, and packaging line systems
Services:
- Serialization setup, commissioning, aggregation, and decommissioning
- Regulatory compliance support and reporting workflows
- Supply chain visibility dashboards and event analytics
- Exception triage, investigation routing, and documentation
- Environmental sensor integration and threshold management
Contact Information:
- Website: rfxcel.com
- Address: 12667 Alcosta Blvd, Suite #375 San Ramon, CA 94583
- LinkedIn: www.linkedin.com/company/rfxcel
- Facebook: www.facebook.com/rfxcel
- Twitter: x.com/rfXcel
- Phone Number: +39-030-7283500
- Email: info@antaresvision.com
9. EviView
EviView is a digital daily management system built for plant operations and production teams in regulated environments. The platform standardizes shift handovers, tiered meetings, and action tracking, keeping frontline updates tied to real-time KPIs and source systems. Centralized views reduce missed handoffs and scattered notes, while structured checklists, ownership, and timestamps create a clear audit trail for shop-floor decisions.
For pharmaceutical manufacturing, the system supports GMP-aligned routines by linking events, tasks, and metrics in a consistent format. Integration with enterprise data sources enables timely escalation and follow-up, and role-based access keeps information routed to the right team. The result is a predictable rhythm for production, maintenance, and quality activities that makes inspection prep less disruptive and keeps evidence organized.
Key Highlights:
- Standardized digital shift handover with traceable actions
- Tiered reporting to align production, quality, and leadership
- Real-time KPI views connected to enterprise data sources
- Task ownership, timestamps, and audit trails for GMP routines
- Configurable workflows to mirror existing site procedures
Services:
- Digital logbooks and meeting boards for shift coordination
- KPI capture, variance alerts, and escalation workflows
- Action item assignment with due dates and follow-up tracking
- Integration with MES, ERP, data lakes, and identity providers
- Site templates for audits, walkthroughs, and routine checks
Contact Information:
- Website: www.eviview.com
- Address: EviView Limited, 74 South Mall, Cork City,Ireland, T12 F3FD
- Phone Number: +353 (0)21 242 7026
- Email: info@eviview.com
10. cyberCTRL
cyberCTRL is a managed cybersecurity and privacy solution that combines governance, risk, and compliance with security operations. The platform packages monitoring, detection, and response with policy and control management, creating a unified environment for evidence, exceptions, and remediation. Open integration supports existing security tools, while program components cover identify, protect, detect, respond, and recover functions in one subscription.
For pharmaceutical organizations, the approach aligns security controls with compliance objectives such as access governance, incident documentation, and third-party oversight. Architecture options include single-tenant environments with SSO integration, and program operations include continuous updates, playbooks, and reporting suitable for audits. The structure reduces tool sprawl and keeps technical safeguards mapped to documented controls and outcomes.
Key Highlights:
- Unified cybersecurity operations with embedded GRC functions
- Continuous monitoring, detection, and response with playbooks
- Control mapping, evidence collection, and audit-ready reports
- Single-tenant deployment options with SSO and policy enforcement
- Open integration to align existing security and IT tools
Services:
- Managed detection and response with incident handling
- Governance, risk, and compliance documentation and reviews
- Control assessments, gap tracking, and remediation workflows
- Third-party and vendor risk coordination with reporting
- Program dashboards for security posture and audit support
Contact Information:
- Website: cyberctrl.net
- Phone Number: (800) 982-3332×747
- Email: cyber@tmgr.com
Conclusion
Pharmaceutical compliance software ties together processes that once lived in separate silos. Instead of treating documentation, risk tracking, or security checks as side tasks, these systems embed them directly into daily operations. Tools like rfxcel, EviView, and cyberCTRL highlight how different focus areas-supply chain traceability, production management, and cybersecurity-can each play a part in keeping compliance consistent and auditable.
The broader point is that compliance is no longer just a reporting exercise. It is an operational layer that supports visibility, accountability, and readiness across the entire business. The choice of platform depends on where the primary challenges lie, but the outcome is similar: fewer manual gaps, clearer records, and processes that can stand up to regulatory scrutiny without slowing down the work itself.