Get the Big Picture view of campus safety
CampusOptics is a cross-functional EH&S platform designed specifically for institutions of higher education. CampusOptics was created to help campus safety professionals improve collaboration, reduce institutional risk and enhance safety culture.
An EH&S solution that is as Mobile as you are
EH&S Professionals are almost never at their desks, which is why CampusOptics offers a mobile app for both IOS and Android devices to support on-the-go access to chemical inventory, hazardous waste containers, inspections, safety assets, incident records and emergency plans.
Barcode & QR code scanning
Use your device’s camera to scan bar/QR codes for key information on safety assets or chemical containers.
In-App Photos and Video
Quickly associate pictures and videos to assets, chemical containers, safety issues and inspection reports.

Talk-to-Text
Streamlin
Access Documents
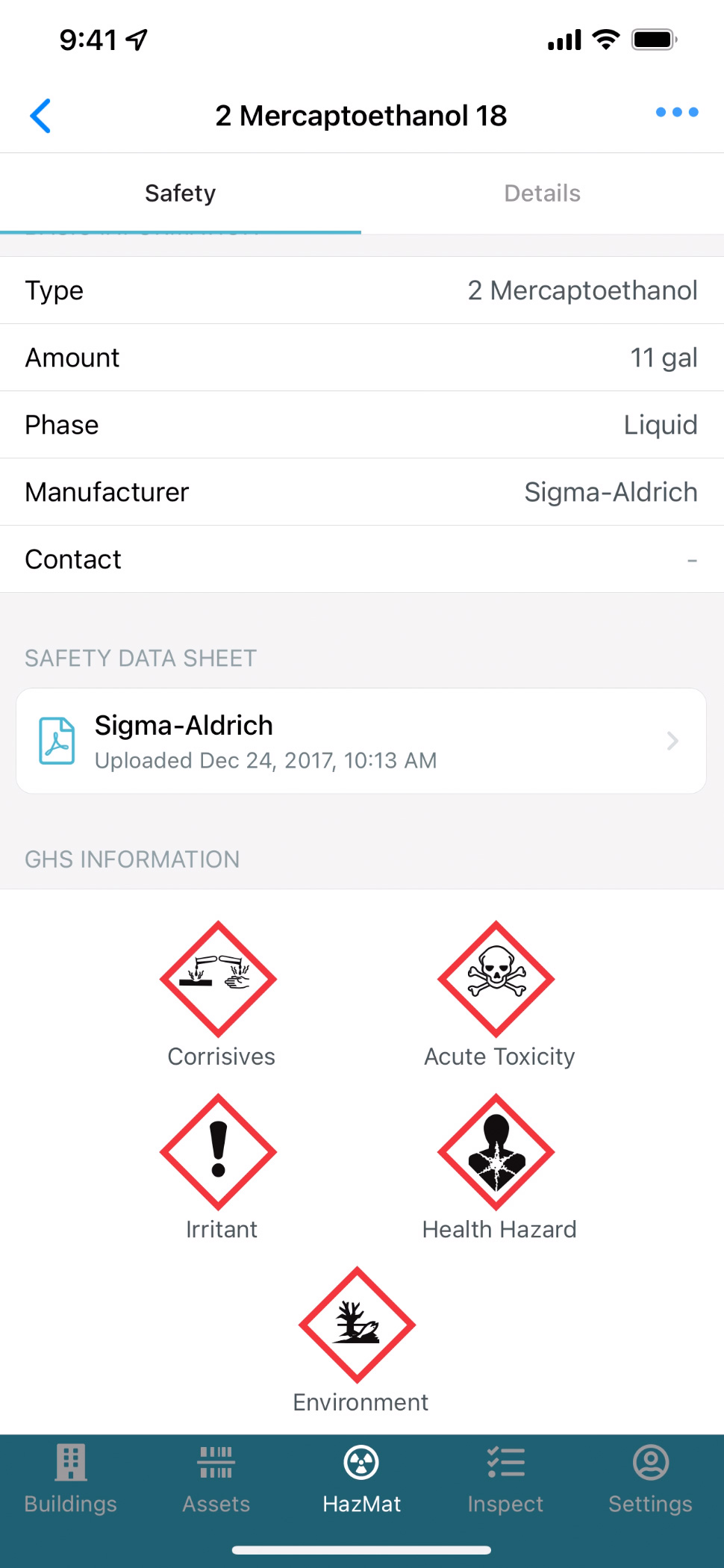
Access key documents on your mobile device, including Safety Data Sheets, emergency plans, floor plans and product documentation.

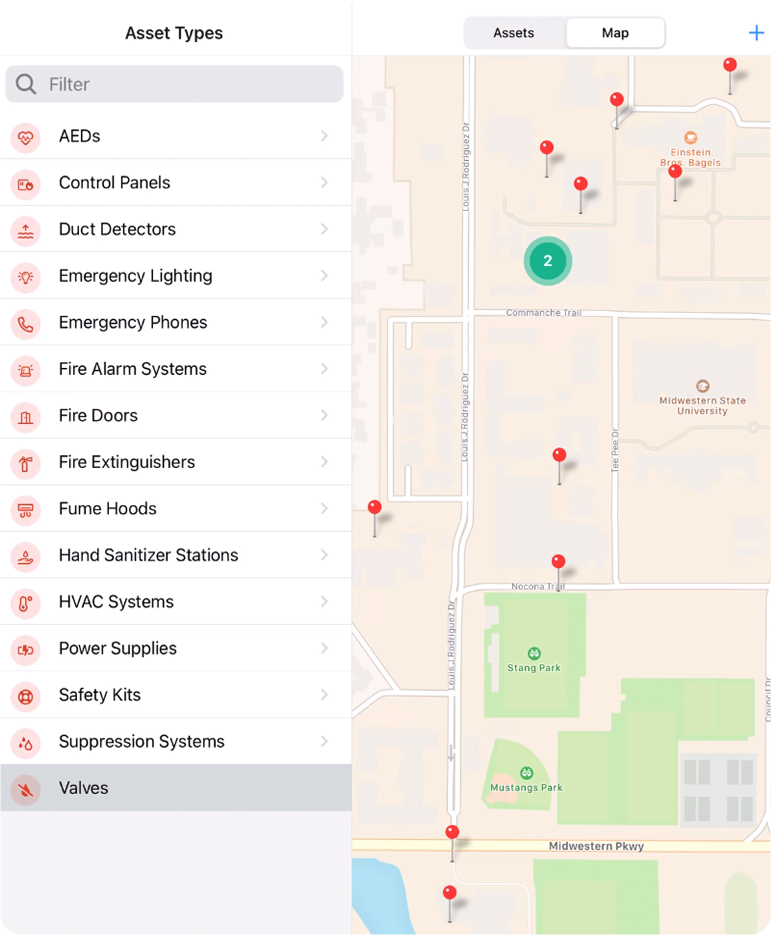
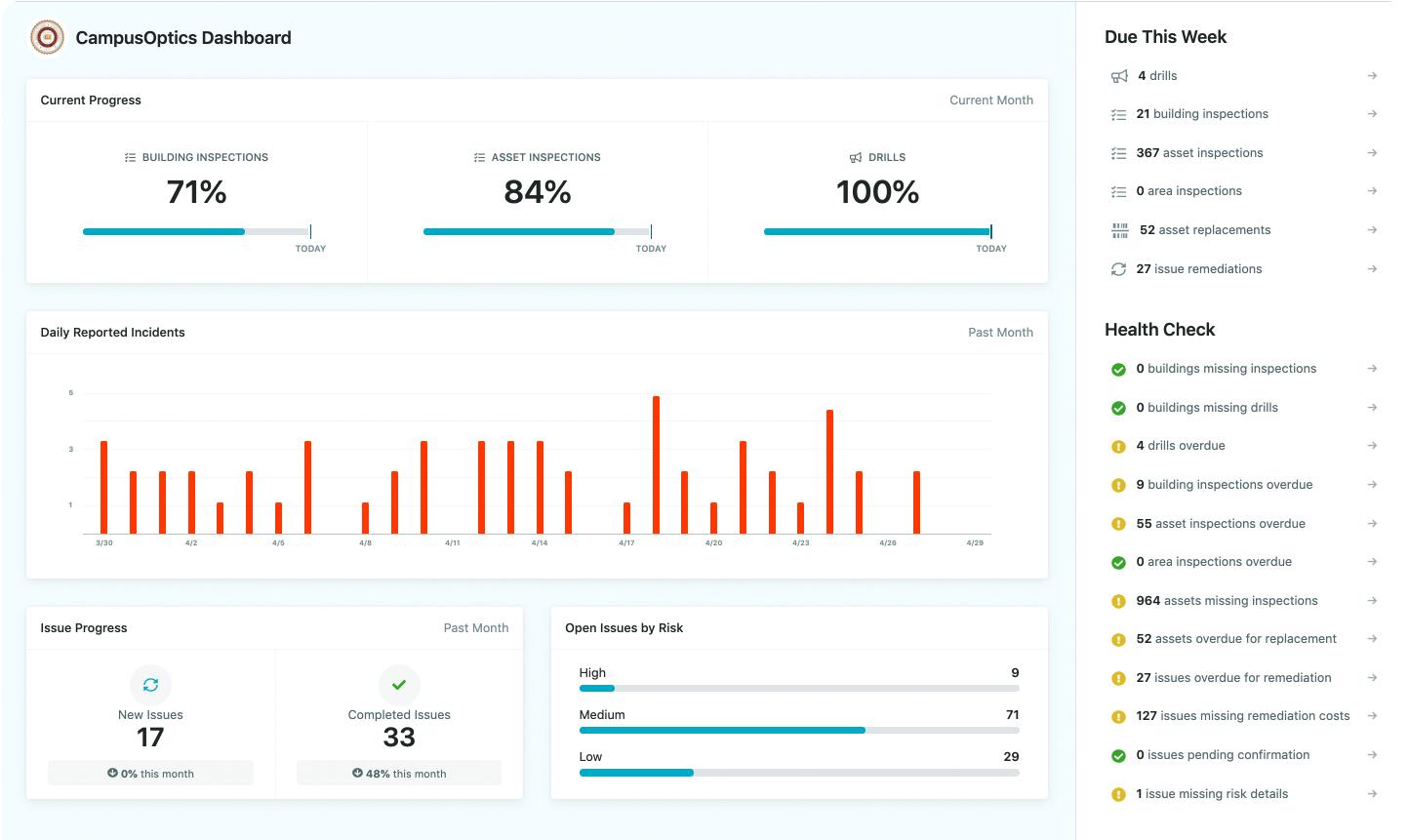
Visualize your data
View the location of safety assets, chemical containers, incidents and safety related issues across campus. Generate public facing maps of key assets like AED’s and Blue Phones.
Locations can be mapped automatically via import or you can log coordinates using your mobile device.
Get the Big Picture view of campus safety
CampusOptics is a cross-functional EH&S platform designed specifically for institutions of higher education. CampusOptics was created to help campus safety professionals improve collaboration, reduce institutional risk and enhance safety culture.
Navigating compliance in life sciences is no small feat. With regulations constantly evolving and high stakes like patient safety and product quality on the line, the right software solutions are more than just tools-they’re essential partners in keeping your processes streamlined and letting you focus on innovation. Here’s a rundown of some top-tier options for 2025, offering a range of solutions tailored to diverse needs, whether you’re managing a campus lab or a global pharma operation.
1. CampusOptics
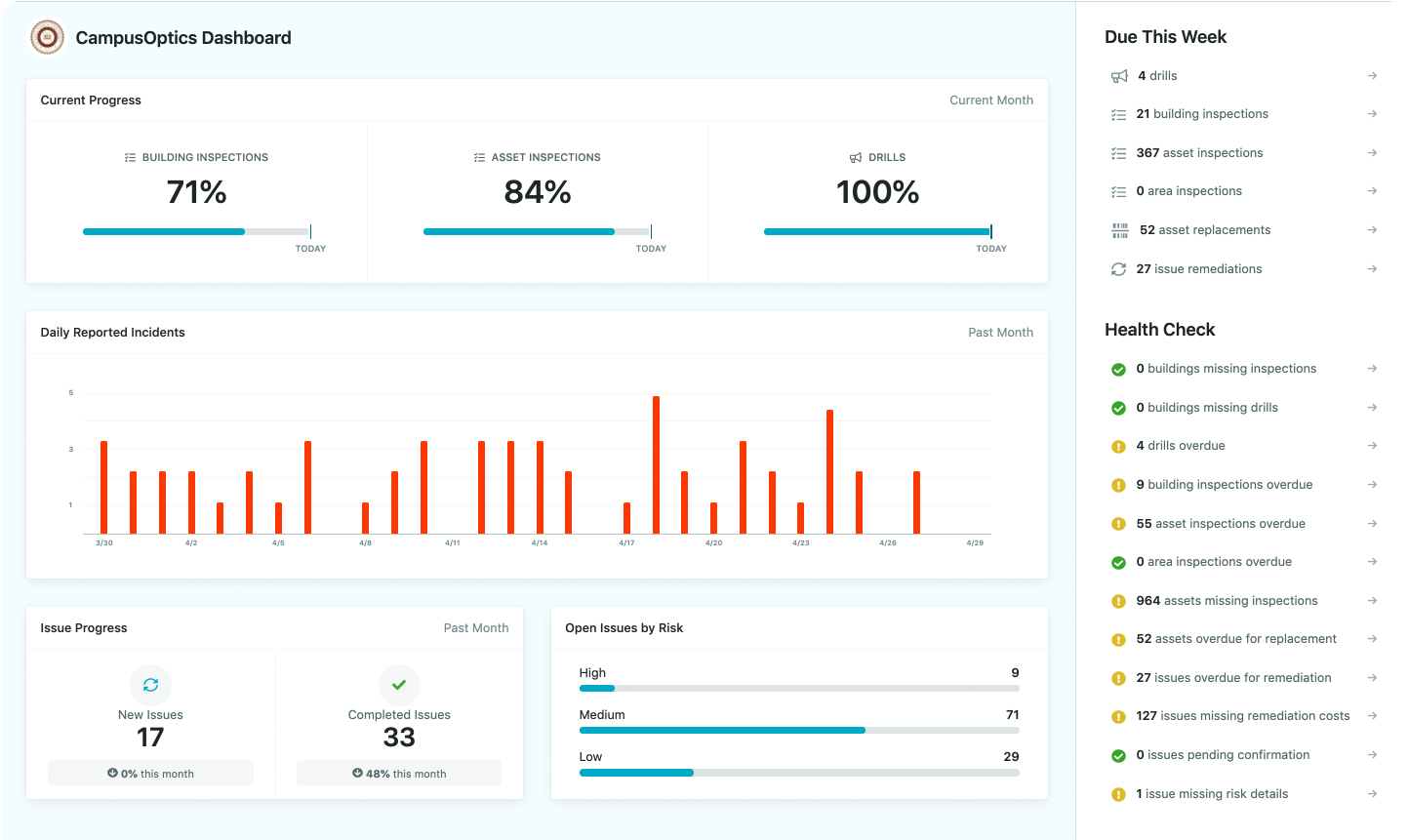
We built CampusOptics as a cross-functional environmental, health, and safety (EHS) platform tailored for higher education institutions, including those with life sciences programs. Our system focuses on managing chemical inventories, hazardous waste, inspections, and incident records, helping teams comply with regulations like OSHA and EPA while fostering a safer campus. It’s designed to support collaboration across departments, ensuring everyone from lab managers to safety officers can access critical data.
Our mobile app lets users handle tasks on the go, with features like barcode scanning and talk-to-text for logging inspections or incidents. We also provide tools for visualizing safety assets and generating maps, which is handy for tracking compliance across sprawling campuses. The platform integrates with single sign-on (SSO) systems, making it easier to manage permissions and keep data secure.
Key Highlights:
- Manages chemical inventories, hazardous waste, and incident records.
- Supports OSHA and EPA compliance for life sciences environments.
- Offers a mobile app with barcode scanning and talk-to-text features.
- Provides data visualization and mapping for safety assets.
- Integrates with SSO for secure, configurable user access.
Who it’s best for:
- Higher education institutions with life sciences labs.
- EHS teams needing mobile tools for compliance and safety.
- Safety officers managing chemical and incident data.
- Campuses seeking collaborative, secure compliance solutions.
Contact Information:
- Website: www.campusoptics.com
- Phone: (888)748-7652
- Email: contact@campusoptics.com
- Address: 7951 Shoal Creek Blvd., Suite 275, Austin, TX 78757
- App Store: apps.apple.com/us/app/campusoptics
- Google Play: play.google.com/store/apps/campusoptics.app
2. MasterControl
MasterControl is like the Swiss Army knife of compliance software for life sciences. It pulls together quality management (QMS), manufacturing execution (MES), and asset management (CMMS) into one slick package. They lean hard into AI to make tasks like document control, training, and production records a breeze. If you’re dealing with GxP workflows, this one’s got your back.
It’s built to meet heavy-hitters like FDA 21 CFR Part 11 and ISO 42001, so your data’s locked down tight. You’ve got tools for electronic batch records (EBR), device history records (eDHR), logbooks, and audit management—basically everything to keep the regulators off your case. Plus, it’s flexible enough to keep up with your innovation without slowing you down.
Key Highlights:
- Combines QMS, MES, and CMMS for comprehensive compliance management.
- Uses AI to streamline document control, training, and production workflows.
- Supports FDA 21 CFR Part 11 and ISO 42001 for regulatory compliance.
- Includes tools for EBR, eDHR, logbooks, and asset lifecycle tracking.
- Offers flexible, digital solutions for quality events and audits.
Who it’s best for:
- Life sciences companies needing integrated quality and manufacturing tools.
- Organizations focused on digitalizing GxP workflows and production records.
- Teams managing complex assets alongside compliance requirements.
- Firms prioritizing FDA and ISO compliance with customizable configurations.
Contact Information:
- Website: www.mastercontrol.com
- Phone: +1 866 747 8767
- Email: info@mastercontrol.com
- Address: MasterControl Solutions, Inc., 6350 South 3000 East, Salt Lake City, UT 84121
- LinkedIn: www.linkedin.com/company/mastercontrol
- Facebook: www.facebook.com/mastercontrolinc
- Twitter: x.com/MCMasterControl
3. Case IQ
Case IQ offers a platform focused on compliance and risk management, particularly for incident reporting and case management in life sciences settings. Their system uses AI to spot patterns, automate workflows, and generate case reports, making it easier to handle incidents from start to finish. Features like a secure whistleblower hotline and compliance monitoring help organizations manage risks proactively.
Tools include omni-channel case intake, approvals and disclosures, and third-party due diligence, all centralized in one system. They provide data-driven insights through analytics and a risk engine that flags anomalies in financial data, which is useful for maintaining compliance across global operations. This platform works well for companies needing strong incident and risk management systems.
Key Highlights:
- Provides a secure, anonymous whistleblower hotline for incident reporting.
- Uses AI-driven Summarization Copilot for quick, consistent case reports.
- Includes compliance monitoring with fraud detection and machine learning.
- Supports approvals, disclosures, and third-party risk management.
- Offers configurable workflows and analytics for efficient case handling.
Who it’s best for:
- Companies focused on workplace incident reporting and ethics compliance.
- Organizations needing automated, data-driven risk management solutions.
- Teams managing third-party relationships and disclosure processes.
- Firms looking for streamlined incident intake and resolution workflows.
Contact Information:
- Website: www.caseiq.com
- Phone: 18004656089
- Email: sales@caseiq.com
- Address: 300 March Road, Suite 501, Ottawa, Ontario K2K 2E2, Canada
- LinkedIn: www.linkedin.com/company/caseiq
- Facebook: www.facebook.com/caseiq
- Twitter: x.com/caseiqsoftware
4. SoftExpert
SoftExpert’s got a cloud-based platform that’s like a command center for compliance, quality, and risk management. They’ve got modules for quality management (QMS), enterprise content management (ECM), and governance, risk, and compliance (GRC). AI helps automate stuff like document control, audits, and performance tracking, so you’re not drowning in paperwork.
It supports standards like ISO 9001 and FDA 21 CFR Part 11, with real-time risk monitoring and supplier management tools. They’ve also got product lifecycle tracking and ESG initiatives baked in, which is great for staying audit-ready across global operations. It’s a one-stop shop for teams with a lot on their plate.
Key Highlights:
- Centralizes QMS, ECM, and GRC in one platform.
- Handles ISO 9001, FDA 21 CFR Part 11, and more.
- AI automates document control and audits.
- Tracks suppliers and product lifecycles.
- Real-time analytics for risks and performance.
Who it’s best for:
- Life sciences firms needing a broad, integrated compliance platform.
- Organizations managing multiple regulatory standards across global operations.
- Teams looking for cloud-based automation and real-time insights.
- Companies handling supplier lifecycles and ESG compliance alongside quality.
Contact Information:
- Website: www.softexpert.com
- Phone: +1 (321) 442-7145
- Email: sales@softexpert.com
- Address: 2121 S Hiawassee Rd, Orlando, FL 32835, USA
- LinkedIn: www.linkedin.com/company/softexpert-software
- Facebook: www.facebook.com/SoftExpertSoftwareforPerformanceExcellence
- Twitter: x.com/softexpert
- Instagram: www.instagram.com/softexpert
5. ETQ Reliance
ETQ Reliance provides a cloud-based quality management system (QMS) tailored for life sciences, focusing on automating compliance tasks like complaints handling and regulatory submissions. Their platform includes applications for managing customer complaints, electronic signatures, and system validation, all designed to meet FDA 21 CFR Part 11 and Annex 11 requirements. It’s built to adapt to evolving business needs through customizable modules, which makes it easier to align with specific workflows.
Tools help track complaints, support electronic Medical Device Reporting (eMDR), and streamline validation processes to minimize disruptions. They use analytics and automation to enhance decision-making and supplier quality, offering a practical solution for organizations looking to integrate quality management into their broader operations.
Key Highlights:
- Automates complaints handling and eMDR for regulatory compliance.
- Supports FDA 21 CFR Part 11 and Annex 11 with e-signature tracking.
- Offers configurable modules for flexible quality management.
- Includes analytics and automation for decision-making and supplier quality.
- Provides validation tools to reduce disruptions in compliance processes.
Who it’s best for:
- Life sciences firms focused on complaints and regulatory submissions.
- Organizations needing a flexible QMS with FDA compliance support.
- Teams aiming to integrate quality management with business processes.
- Companies seeking automated validation and supplier quality tools.
Contact Information:
- Website: www.etq.com
- Phone: +1 (844) 293-3001
- Address: 305 Intergraph Way, Madison, AL 35758
- LinkedIn: www.linkedin.com/company/etq
- Facebook: www.facebook.com/etqllc
- Twitter: x.com/etqsoftware
- Instagram: www.instagram.com/etqsoftware
6. UL Solutions
UL Solutions offer a suite of software and services centered on compliance, safety, and sustainability for life sciences and other industries. Their platform includes tools for managing regulatory requirements, supply chain compliance, and sustainability initiatives, paired with auditing and certification services. They focus on helping organizations navigate complex regulations while ensuring product safety and performance.
Software supports certification tracking, audits, and verification of environmental claims, with an emphasis on real-time data and global standards like FDA and ISO. They also provide learning and development resources to keep teams informed about regulatory changes, making it a good fit for companies with diverse compliance needs.
Key Highlights:
- Supports certification, auditing, and supply chain compliance.
- Aligns with FDA and ISO standards for regulatory compliance.
- Offers tools for sustainability and environmental claim verification.
- Includes learning resources for staying updated on regulations.
- Provides real-time data for global compliance management.
Who it’s best for:
- Life sciences firms with broad safety and sustainability goals.
- Organizations needing certification and auditing support.
- Teams managing global supply chains and regulatory requirements.
- Companies seeking training tools alongside compliance software.
Contact Information:
- Website: www.ul.com
- Phone: +1.510.210.3333
- Email: BEN.Softlines.Customer.Service@crs.ul.com
- Address: 1000 Broadway, Units 200 D and 200 E, Oakland, California, 94607
- LinkedIn: www.linkedin.com/company/ulsolutions
- Facebook: www.facebook.com/ULSolutionsGlobal
- Twitter: x.com/UL_Solutions
7. SAI360
SAI360 provides a governance, risk, and compliance (GRC) platform that integrates ethics, risk management, and compliance for life sciences organizations. Their system uses configurable modules to handle risks, compliance training, and policy administration, with analytics to keep up with regulatory changes. It’s designed to connect processes across departments, reducing silos and improving visibility.
Tools offer risk insights, behavioral analytics, and compliance program management, helping teams tackle regulatory and ethical challenges holistically. They focus on unifying data across functions to support better decision-making, which works well for companies looking for a comprehensive approach to compliance.
Key Highlights:
- Integrates GRC with ethics, risk, and compliance training.
- Offers configurable modules for tailored risk and compliance programs.
- Uses analytics to adapt to regulatory and risk changes.
- Unifies processes to reduce departmental silos.
- Supports compliance with global regulatory standards.
Who it’s best for:
- Life sciences firms needing integrated GRC and ethics solutions.
- Organizations managing complex risk and compliance programs.
- Teams seeking analytics-driven insights for decision-making.
- Companies aiming to unify compliance across multiple functions.
Contact Information:
- Website: www.sai360.com
- Phone: (312) 546-4500
- Address: 205 West Wacker Drive, Suite 1800, Chicago, IL 60606
- LinkedIn: www.linkedin.com/company/sai360
8. SimplerQMS
SimplerQMS offers a cloud-based quality management system (QMS) tailored for life sciences, focusing on automating processes like document control, training, and CAPA management. Their platform integrates all QMS modules into a single solution, supporting compliance with standards like FDA 21 CFR Part 11, ISO 13485, and GxP. It’s designed to streamline workflows and reduce manual tasks, with features like automated audit trails and Microsoft Office 365 integration.
The system comes pre-validated per GAMP5 guidelines, saving time on manual validation efforts. They provide tools for managing deviations, complaints, and risk, alongside training and equipment management, all connected in a closed-loop system to keep quality and compliance data centralized. This setup works well for teams needing a straightforward, all-in-one compliance tool.
Key Highlights:
- Integrates all QMS modules for document control, training, and CAPA.
- Supports FDA 21 CFR Part 11, ISO 13485, and GxP standards.
- Offers pre-validated software with GAMP5-compliant documentation.
- Includes Microsoft Office 365 integration for seamless document editing.
- Provides tools for deviation, complaint, and risk management.
Who it’s best for:
- Life sciences firms needing an all-in-one, pre-validated QMS.
- Teams looking to streamline document and training processes.
- Organizations managing medical device or pharmaceutical compliance.
- Companies wanting integrated workflows with minimal setup effort.
Contact Information:
- Website: simplerqms.com
- Phone: +18888309807
- Address: 600 B St, San Diego, CA, 92101, United States
- LinkedIn: www.linkedin.com/company/simplerqms
9. Compliance Solutions
Compliance Solutions provide consultancy-driven software and services focused on regulatory compliance for life sciences, particularly medical devices and biologics. Their platform supports navigating FDA processes, CE marks, and international submissions, with tools for quality management system (QMS) implementation and maintenance. They emphasize practical, hands-on solutions tailored to specific project needs.
Their system helps companies meet standards like FDA, MHRA, and ICH requirements, offering support for audits and regulatory approvals. They focus on flexibility, adapting to the varying demands of startups to large organizations, and provide expertise in areas like in-vitro diagnostics and blood establishments. This approach suits firms needing both software and expert guidance.
Key Highlights:
- Supports FDA, MHRA, and ICH regulatory requirements.
- Offers tools for QMS implementation and maintenance.
- Provides expertise in medical devices, IVDs, and biologics.
- Facilitates international regulatory submissions and audits.
- Adapts to varying project needs with tailored solutions.
Who it’s best for:
- Medical device and biologics firms needing regulatory guidance.
- Organizations seeking flexible QMS and compliance support.
- Startups and large companies navigating FDA or CE processes.
- Teams requiring hands-on consultancy alongside software tools.
Contact Information:
- Website: cslifesciences.com
- Phone: +1305 960 7262
- Email: edwin@cslifesciences.com
- Address: CS Lifesciences Europe Limited, The Black Church, St. Mary’s Place, Dublin 7, Dublin, D07P4AX, Ireland
- LinkedIn: www.linkedin.com/company/compliance-solutions-life-sciences-ltd
10. GAN Integrity
GAN Integrity delivers a compliance platform centered on ethics, risk, and regulatory management for life sciences companies. Their system integrates tools for third-party risk management, disclosure management, and anti-bribery compliance, supporting regulations like FCPA, GDPR, and the EU Whistleblower Directive. It’s designed to centralize data and provide clear visibility into compliance risks.
The platform offers workflows for managing conflicts of interest, supply chain due diligence, and compliance training, with automated approvals and policy management. They use analytics to help teams stay ahead of regulatory changes, making it a good fit for organizations with complex, global compliance needs.
Key Highlights:
- Integrates ethics, risk, and compliance management tools.
- Supports FCPA, GDPR, and EU Whistleblower Directive compliance.
- Offers third-party risk management and disclosure workflows.
- Provides analytics for monitoring regulatory and risk changes.
- Centralizes data for better visibility across compliance programs.
Who it’s best for:
- Life sciences firms with global ethics and compliance needs.
- Organizations managing third-party risks and disclosures.
- Teams needing automated workflows for anti-bribery compliance.
- Companies seeking data-driven insights for regulatory management.
Contact Information:
- Website: www.ganintegrity.com
- Address: 228 Park Ave S, PMB 44939,New York, NY 10003, United States of America
- LinkedIn: www.linkedin.com/company/ganintegrity
11. Springs
Springs provide an AI-powered compliance platform tailored for life sciences, focusing on real-time regulatory monitoring and workflow automation. Their system uses generative AI to track global regulations, analyze impacts, and integrate with existing SOPs and CAPAs, helping teams stay audit-ready. It’s designed to adapt to specific business needs, offering customizable workflows and centralized data management.
Tools include a regulatory library, horizon scanning, and AI-driven gap analysis to identify compliance risks. They support standards like FDA, EMA, and ISO, with features for automated alerts and audit preparation. This platform suits organizations needing dynamic, tech-forward solutions to handle complex regulatory landscapes.
Key Highlights:
- Uses AI to monitor global regulations and provide real-time alerts.
- Offers customizable workflows for SOPs, CAPAs, and compliance tasks.
- Includes a regulatory library with full traceability to internal policies.
- Provides AI-driven gap analysis to identify compliance vulnerabilities.
- Integrates with existing systems for seamless data management.
Who it’s best for:
- Life sciences firms needing real-time regulatory monitoring.
- Teams seeking customizable, AI-powered compliance workflows.
- Organizations managing complex global standards like FDA and EMA.
- Companies looking for automated audit preparation tools.
Contact Information:
- Website: springsapps.com
- Phone: +34666845262
- Email: contact@springsapps.com
- LinkedIn: www.linkedin.com/company/springs—custom-web-development
- Facebook: www.facebook.com/springsdev
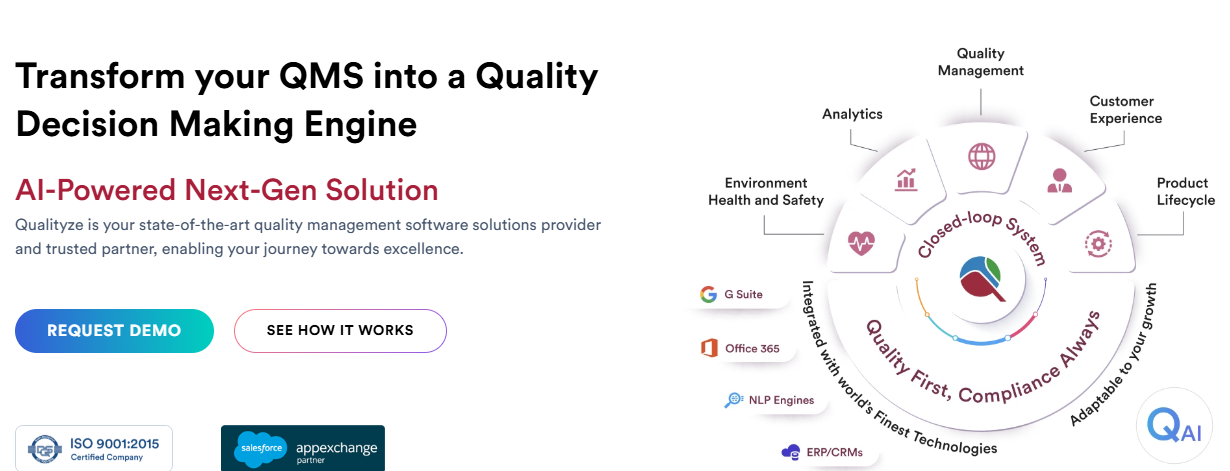
12. Qualityze
Qualityze offers a cloud-based enterprise quality management system (EQMS) for life sciences, designed to streamline quality and compliance processes. Their platform includes tools for document control, risk management, and supplier quality, with pre-configured workflows to meet FDA 21 CFR Part 11, EU MDR, and ISO standards. It’s built to ensure data integrity and traceability across operations.
The system supports CAPA management, audit readiness, and supplier performance tracking, with AI-powered features to enhance decision-making. They focus on integrating with existing systems, making it easier for teams to maintain compliance while optimizing quality control. This platform works well for organizations balancing regulatory demands with operational efficiency.
Key Highlights:
- Supports FDA 21 CFR Part 11, EU MDR, and ISO standards.
- Includes tools for document control, CAPA, and supplier management.
- Offers AI-powered analytics for proactive risk mitigation.
- Provides pre-configured workflows for audit readiness.
- Integrates with existing systems for centralized data management.
Who it’s best for:
- Life sciences firms needing integrated quality and compliance tools.
- Organizations focused on supplier quality and traceability.
- Teams seeking AI-driven insights for risk and audit management.
- Companies aiming for seamless integration with existing systems.
Contact Information:
- Website: www.qualityze.com
- LinkedIn: www.linkedin.com/company/qualityze-inc
- Facebook: www.facebook.com/Qualityze
- Twitter: x.com/Qualityze
13. Baker Tilly complianceNOW
complianceNOW is a tool of Baker Tilly, offering a suite of technology-driven compliance solutions tailored for the life sciences industry. It focuses on managing interactions with healthcare professionals (HCPs) and ensuring global transparency reporting. The platform includes tools for fair market value (FMV) compensation, key opinion leader (KOL) tiering, and spend transparency, designed to streamline compliance tasks across jurisdictions.
Integrating advisory services with user-friendly tools, complianceNOW supports single sign-on (SSO) and scalable workflows. It embeds compliance controls into processes like HCP engagements and stewardship funding, aligning with global regulatory requirements. This platform is ideal for organizations seeking specialized tools for HCP and transparency compliance, backed by Baker Tilly’s expertise in advisory, tax, and assurance services.
Key Highlights:
- Offers tools for FMV compensation and KOL tiering.
- Supports global spend transparency reporting across jurisdictions.
- Includes SSO and scalable workflows for compliance tasks.
- Integrates advisory services with technology-driven solutions.
- Focuses on HCP engagement and stewardship compliance.
Who it’s best for:
- Life sciences firms managing HCP engagements and transparency reporting.
- Organizations needing scalable compliance tools for global operations.
- Teams seeking integrated advisory and technology solutions.
- Companies focused on stewardship and regulatory compliance.
Contact Information:
- Website: www.bakertilly.com
- Phone: +1 (520) 836 8201
- Address: 1115 E Cottonwood Lane, Suite 100, Casa Grande, AZ 85122
- LinkedIn: www.linkedin.com/company/bakertillyus
- Facebook: www.facebook.com/bakertillyus
- Instagram: www.instagram.com/bakertillyus
14. Ideagen
Ideagen offers a governance, risk, and compliance (GRC) platform tailored for life sciences, focusing on quality, safety, and regulatory processes. Their system includes tools for document management, environmental, health, and safety (EHS) processes, and enterprise risk management, supporting compliance with standards like FDA 21 CFR Part 11 and ISO 27001. It’s designed to centralize data and streamline operations across complex regulatory environments.
Their platform supports collaboration, audit management, and supply chain quality assurance, helping teams maintain compliance while improving efficiency. They emphasize secure, FedRAMP-authorized solutions to protect sensitive data, making it a practical choice for organizations dealing with strict regulations and global operations.
Key Highlights:
- Supports FDA 21 CFR Part 11 and ISO 27001 compliance.
- Includes tools for EHS, document management, and risk management.
- Offers secure, FedRAMP-authorized solutions for data protection.
- Facilitates collaboration and audit processes across teams.
- Provides supply chain quality assurance for regulatory standards.
Who it’s best for:
- Life sciences firms needing integrated GRC and EHS solutions.
- Organizations managing global regulatory and cybersecurity requirements.
- Teams seeking secure collaboration and document management tools.
- Companies focused on supply chain quality and audit readiness.
Contact Information:
- Website: www.ideagen.com
- Phone: +44 (0) 1629 699 100
- Address: 1 Mere Way Ruddington, Fields Business Park, Nottinghamshire, NG11 6JS, United Kingdom
- LinkedIn: www.linkedin.com/company/ideagen
- Facebook: www.facebook.com/Ideagensolutions
- Twitter: x.com/Ideagen_
15. AWS
AWS provides a cloud-based platform for life sciences compliance, focusing on automating GxP processes and enhancing security. Their system offers tools for building GxP-compliant environments, with automated traceability and infrastructure control to meet standards like FDA, HIPAA, and ISO. It’s designed to help organizations manage sensitive data and respond to audits efficiently.
Their platform supports a shared responsibility model, where AWS handles infrastructure security while customers manage application-level compliance. They offer extensive compliance certifications and tools for encryption, data privacy, and audit logging, making it a good fit for teams needing scalable, secure cloud solutions.
Key Highlights:
- Automates GxP compliance with tools for traceability and audits.
- Supports FDA, HIPAA, and ISO standards with extensive certifications.
- Offers a shared responsibility model for infrastructure security.
- Provides encryption and data privacy tools for sensitive data.
- Enables scalable cloud environments for global operations.
Who it’s best for:
- Life sciences firms needing secure, cloud-based GxP solutions.
- Organizations managing sensitive data and global compliance.
- Teams seeking automated traceability for audit responses.
- Companies looking for scalable infrastructure with security controls.
Contact Information:
- Website: aws.amazon.com
- LinkedIn: www.linkedin.com/company/amazon-web-services
- Facebook: www.facebook.com/amazonwebservices
- Instagram: www.instagram.com/amazonwebservices
16. Intellect
Intellect delivers an AI-powered quality management system (QMS) for life sciences, focusing on automating processes like document control, calibration, and training. Their platform includes over 30 pre-built apps to support compliance with FDA, ISO, and GxP regulations, with flexible reporting and intelligent search features to streamline workflows.
The system uses AI to analyze trends, identify root causes, and recommend solutions, helping teams reduce the cost of quality. They focus on adaptability, allowing non-technical users to customize the platform, which makes it suitable for organizations needing flexible, user-friendly compliance tools.
Key Highlights:
- Offers over 30 pre-built apps for document control and training.
- Supports FDA, ISO, and GxP compliance with AI-driven analytics.
- Includes intelligent search and flexible reporting features.
- Allows customization by non-technical users for specific needs.
- Focuses on reducing cost of quality through automation.
Who it’s best for:
- Life sciences firms needing customizable, AI-powered QMS tools.
- Teams seeking user-friendly solutions for document and training management.
- Organizations focused on FDA and ISO compliance.
- Companies aiming to reduce quality costs through automation.
Contact Information:
- Website: intellect.com
- Phone: +1 (800) 558-6832
- Email: sales@intellect.com
- Address: 1601 Centinela Avenue, Los Angeles, CA 90302
- LinkedIn: www.linkedin.com/company/interneer
- Facebook: www.facebook.com/IntellectIQ
- Instagram: www.instagram.com/intellect.iq
17. MetricStream
TheyMetricStream provides an AI-driven governance, risk, and compliance (GRC) platform for life sciences, focusing on integrating risk management, compliance, and audit processes. Their system automates regulatory updates, policy management, and third-party risk assessments, supporting standards like ISO 27001 and NIST frameworks. It’s designed to give teams real-time visibility into risks and compliance across the enterprise.
Tools include features for continuous audits, cyber risk management, and business continuity planning, helping organizations stay resilient. They emphasize streamlining workflows and reducing manual tasks, which makes it a solid choice for companies managing complex regulatory and risk environments.
Key Highlights:
- Automates regulatory updates and compliance assessments with AI.
- Supports ISO 27001, NIST, and other regulatory frameworks.
- Offers tools for third-party risk and cyber resilience management.
- Provides real-time insights for audits and risk visibility.
- Streamlines policy management and business continuity planning.
Who it’s best for:
- Life sciences firms needing integrated GRC and cyber risk tools.
- Organizations managing complex regulatory and third-party risks.
- Teams seeking automated audits and real-time risk insights.
- Companies focused on enterprise-wide compliance and resilience.
Contact Information:
- Website: www.metricstream.com
- Phone: +1-650-620-2955
- Email: info@metricstream.com
- Address: 6201 America Center Drive, Suite 120, San Jose, CA 95002
- LinkedIn: www.linkedin.com/company/metricstream
- Facebook: www.facebook.com/MetricStream
- Twitter: x.com/metricstream
18. Qualio
Qualio a cloud-based quality management system (QMS) tailored for life sciences startups and scale-ups, focusing on simplifying compliance processes. Their platform includes tools for document control, training, audits, and CAPA management, supporting standards like FDA 21 CFR Part 11, ISO 9001, and ICH guidelines. It’s built to integrate with business-critical applications, creating a unified source for quality data.
The system emphasizes ease of use and collaboration, with features that streamline regulatory workflows and audit preparation. They focus on connecting quality processes across teams, making it a practical option for organizations aiming to embed compliance into their operations without complexity.
Key Highlights:
- Supports FDA 21 CFR Part 11, ISO 9001, and ICH compliance.
- Includes tools for document control, training, and CAPA management.
- Integrates with business applications for unified quality data.
- Streamlines audit preparation and regulatory workflows.
- Focuses on user-friendly collaboration across teams.
Who it’s best for:
- Life sciences startups and scale-ups needing simple QMS tools.
- Teams seeking integrated document and training management.
- Organizations focused on FDA and ISO compliance.
- Companies aiming for collaborative, audit-ready quality processes.
Contact Information:
- Website: www.qualio.com
- Phone: 01 574 3046
- Email: support@qualio.com
- Address: 13-18 City Quay, Dublin D02 ED70, Ireland
- LinkedIn: www.linkedin.com/company/qualiohq
- Twitter: x.com/QualioHQ
- Instagram: www.instagram.com/lifeatqualio
Conclusion
Navigating compliance in the life sciences world is no small task-regulations are always shifting, and the stakes are high with patient safety and product quality on the line. The platforms we’ve explored tackle these challenges in their own ways, whether it’s through AI-driven risk management, streamlined quality processes, smart manufacturing solutions, or campus-focused safety tools. Each offers practical features to help teams stay on top of standards like FDA 21 CFR Part 11, ISO, or OSHA, while making day-to-day work a bit less overwhelming.
What stands out is how these tools reflect the diversity of needs in life sciences, from startups racing to market to sprawling campuses managing lab safety. Choosing the right one depends on your specific goals-whether it’s automating audits, securing supply chains, or keeping chemical inventories in check. It’s less about finding a perfect fix and more about picking a system that fits your team’s workflow and grows with you. Compliance isn’t just checking boxes; it’s about building systems that let you focus on innovation while keeping risks in check.